Multiplexed Influenza Assays to Improve Time to Market
Simplify multivalent influenza vaccine characterization and reduce analytical development time by leveraging lnDevR’s 20 years of experience in the influenza field.
The VaxArray Influenza Advantage
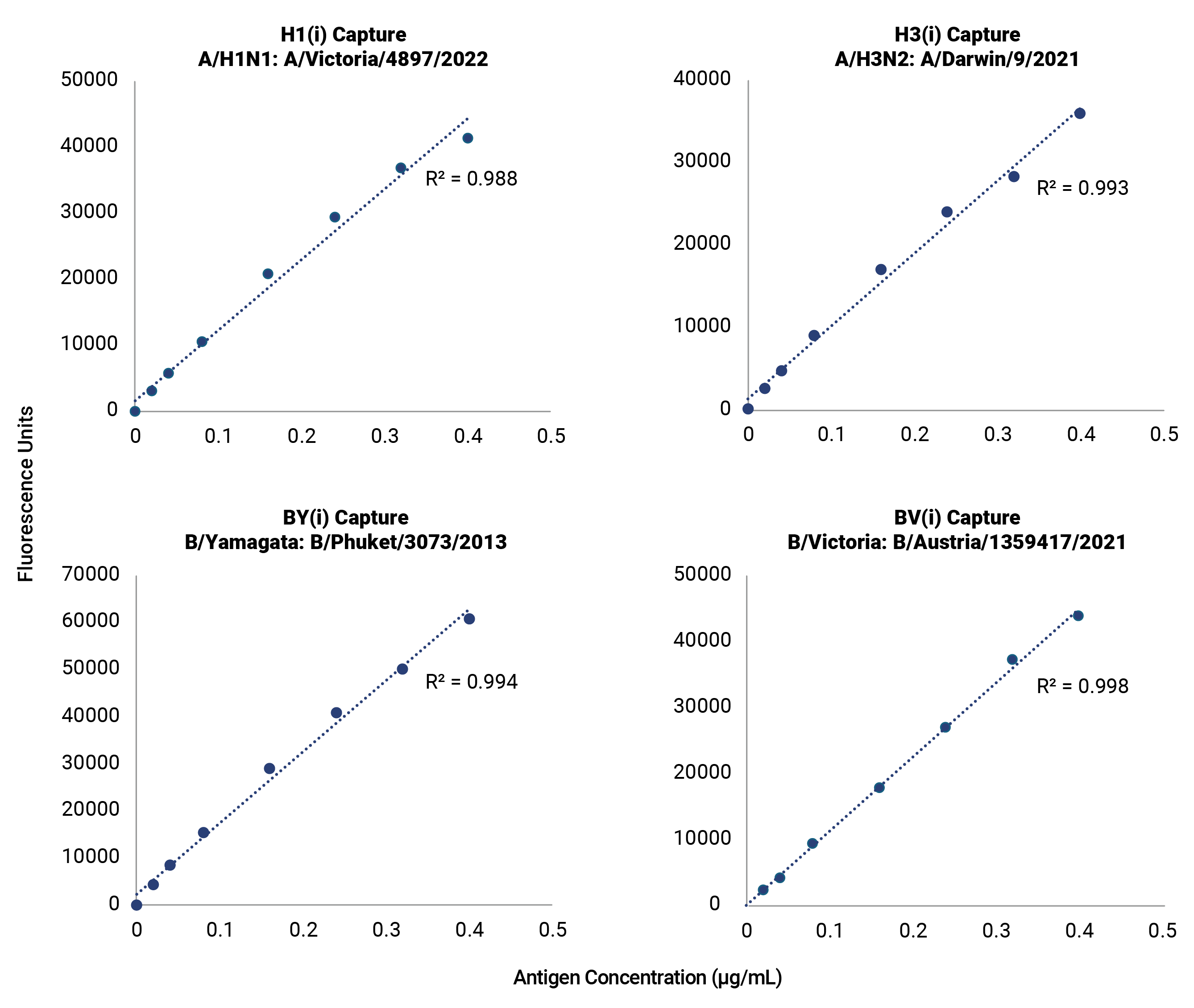
VaxArray® influenza multiplexed immunoassays for subtype-specific detection of seasonal and pandemic hemagglutinin (HA), seasonal neuraminidase (NA), and nucleoprotein (NP) contain a tailored panel of pre-validated capture reagents to facilitate the development, manufacture, and release of vaccines.

- Confirmed reactivity with every World Health Organization (WHO)-recommended strain
- High sensitivity and specificity
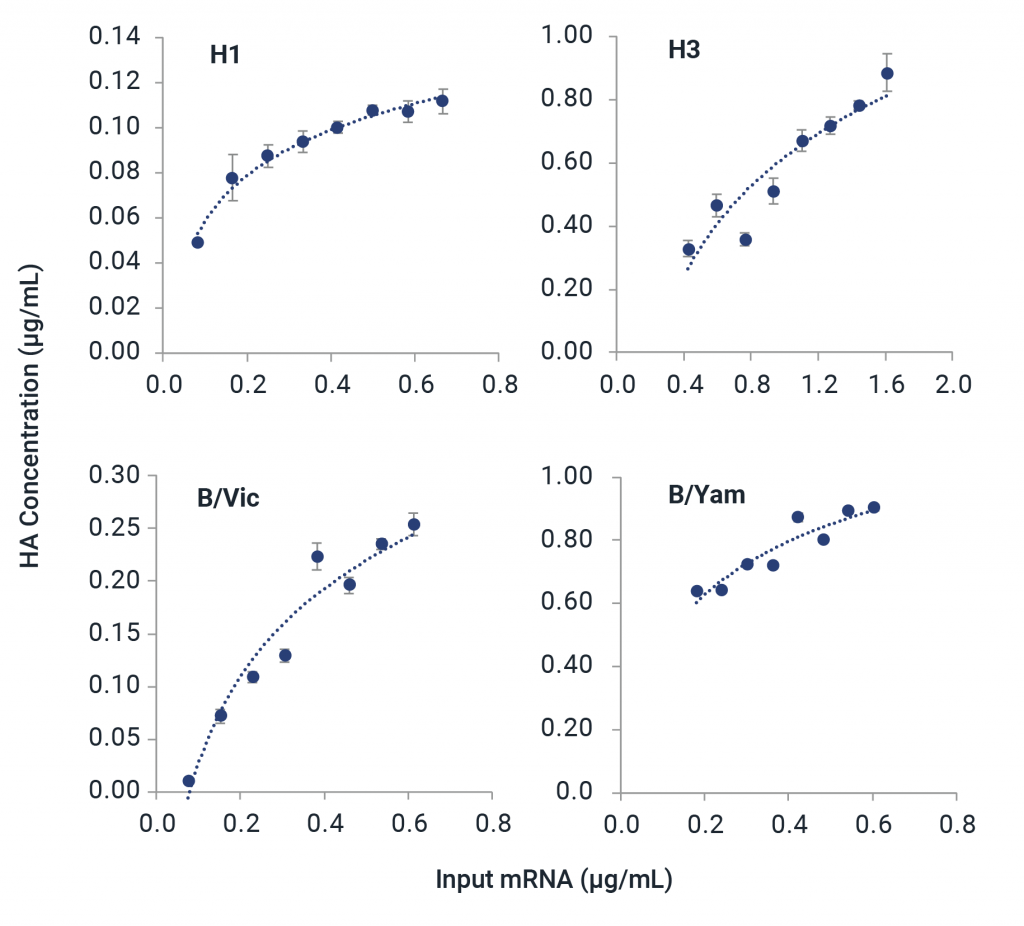
- Validated with wide range of expression systems: egg-based, cell-based, recombinant, and cell-based protein expression with mRNA vaccines
- Multivalent vaccine identity, quantity, and stability testing in one assay
- Simple assay workflow
- Recognized by the FDA and Health Canada for identity testing
- Scanned on our proprietary VaxArray instrument for automated data analysis
Accelerate Vaccine Development with Customized Solutions
Custom Influenza Assays
For a more personalized approach, reach out to our Expert Services Team to develop a custom assay with exclusive access to InDevR’s repository of 300+ strain-specific influenza antibodies or your specific capture reagents.
Custom Assays for Clinical Trials
Accelerate pre-clinical and clinical trial testing by having our Expert Services Team develop an assay to rapidly quantify the antibody response to the specific influenza antigens in your serological samples.
Custom mRNA Assays
Partner with InDevR’s Expert Services Team to eliminate mRNA analytical development bottlenecks with a custom-tailored solution for your mRNA vaccine. The VaxArray Platform provides versatility for characterization of mRNA therapeutics.
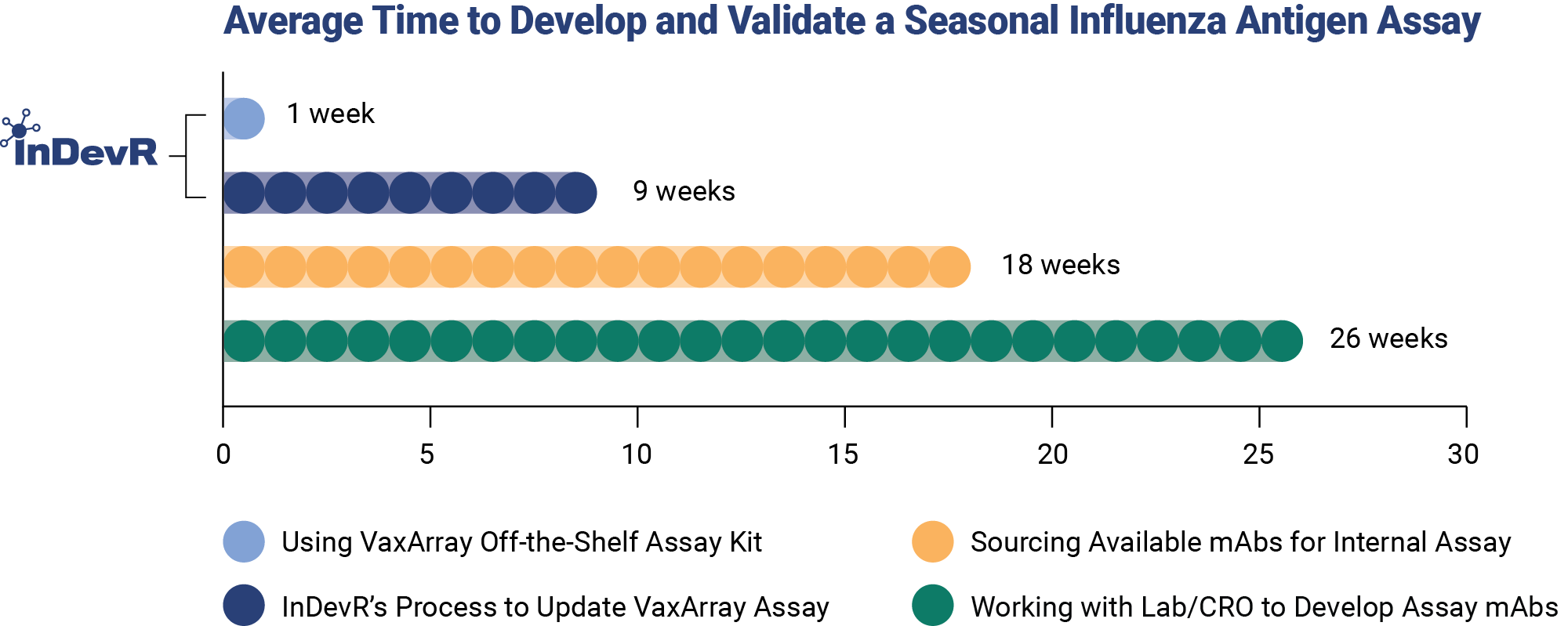
Pre-Validated Assays for Faster Time to Market
The World Health Organization releases influenza strain recommendations semiannually for inclusion in Northern and Southern Hemisphere vaccine formulations. Influenza strains changes often require updates to currently used analytical methods, increasing the analytical burden on influenza vaccine developers and manufacturers.
InDevR does the hard part for you by ensuring reactivity to World Health Organization-recommended strains, eliminating months of analytical development time. Benefit from our growing portfolio of over 300+ subtype-specific influenza monoclonal antibodies for rapid product updates.
Applications
InDevR’s ready-to-go influenza kits for the VaxArray Platform address vaccine manufacturers’ unique challenges by providing pre-validated, off-the-shelf solutions that drastically reduce the validation burden.
- Measure concentration in low antigen content applications, such as microneedle patches
- Streamline seed strain optimization
- Quantify in-process antigen recovery
- Quickly and efficiently explore process improvements
- Track monobulk intermediate stability
- Track stability of all antigen components in multivalent formulation pre- and post-lot release
- Test expression of mRNA constructs during vaccine development
- Measure concentration in low antigen content applications, such as microneedle patches
- Streamline seed strain optimization
- Quantify in-process antigen recovery
- Quickly and efficiently explore process improvements
- Track monobulk intermediate stability
- Track stability of all antigen components in multivalent formulation pre- and post-lot release
- Test expression of mRNA constructs during vaccine development
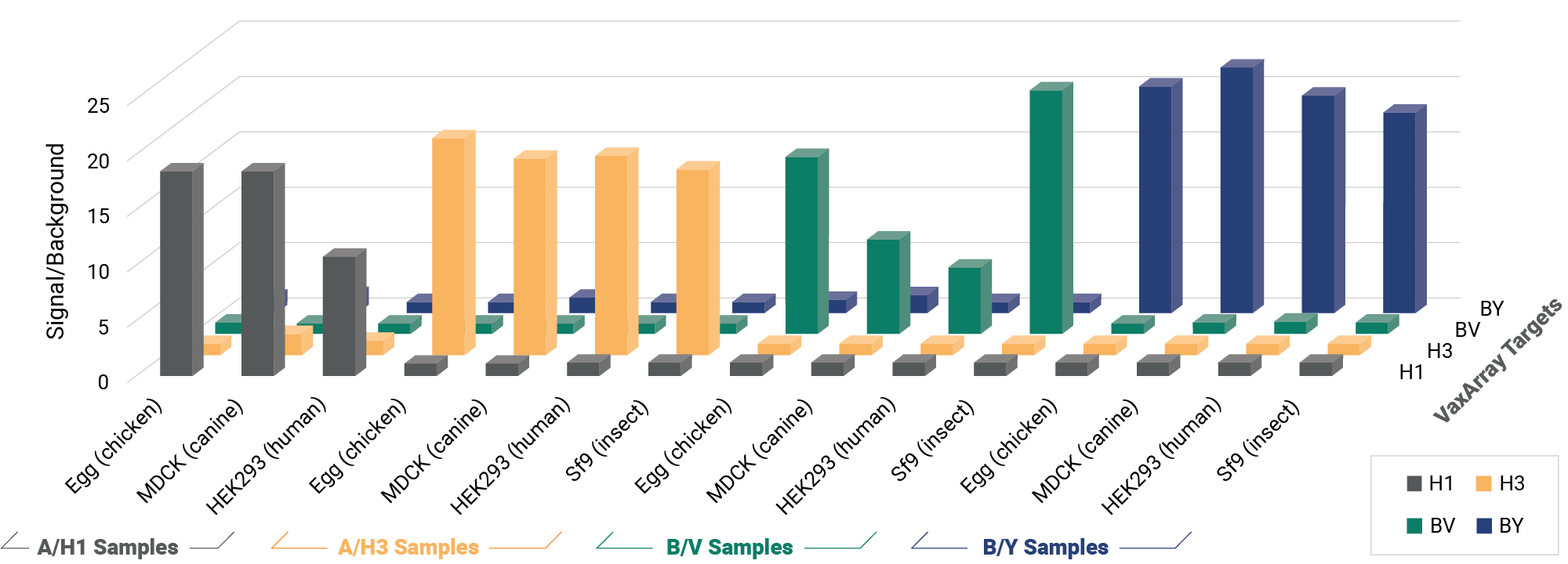
OmniFlu HA
A Versatile Multivalent Hemagglutinin Assay for Identity and Quantity of Seasonal Influenza Subtypes
Improve your time to market with pre-validated detection of hemagglutinin (HA) from influenza subtypes A/H1, A/H3, and B/Victoria and B/Yamagata lineages for every WHO-recommended seasonal strain. The OmniFlu HA Kit is an off-the-shelf, validated solution for seasonal influenza vaccine manufacturers, providing production platform flexibility for hemagglutinin quantification and detection. The novel design of the OmniFlu HA enables comprehensive multivalent detection of influenza HA from a wide range of expression systems, including egg, cell, recombinant, and HA proteins expressed after mRNA-transfection.
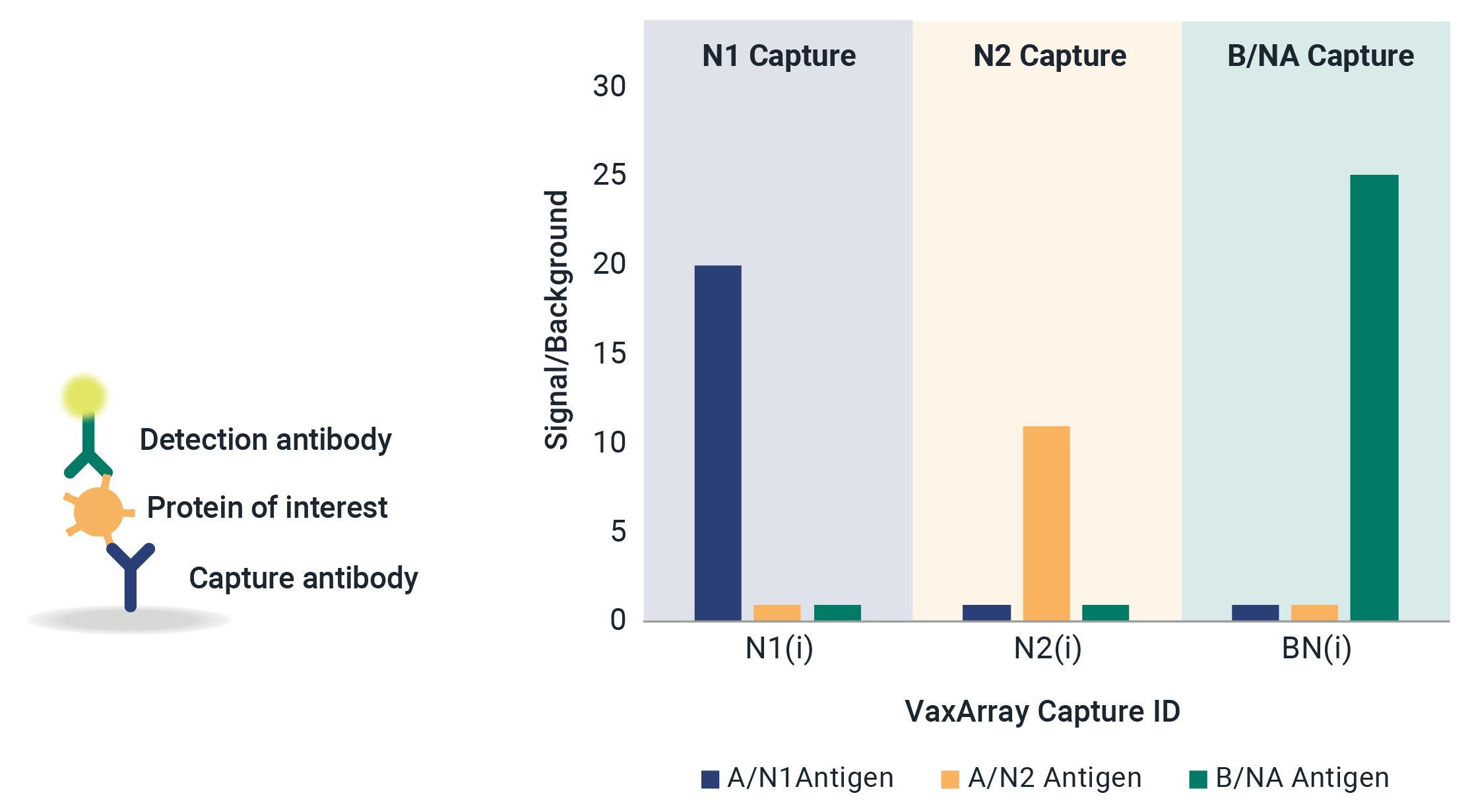
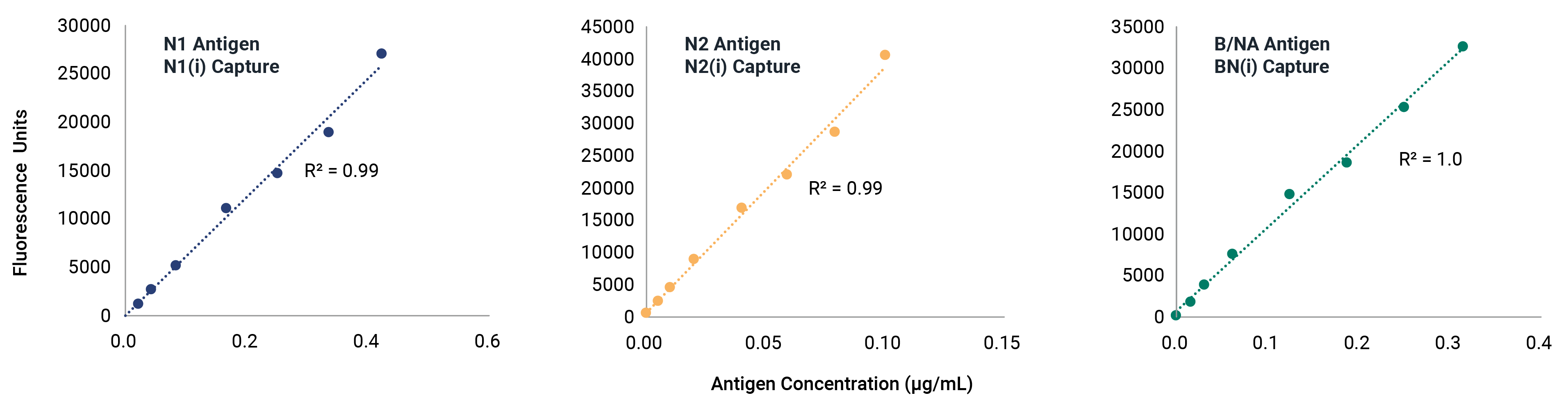
VaxArray Influenza Seasonal Neuraminidase Multivalent Assay
Neuraminidase (NA) identity testing is required for any vaccines expected to contain NA, and NA quantification is an area of active interest for future regulation of whole virus, split virus, or subunit influenza vaccines. Identity and quantification of influenza neuraminidase subtypes N1, N2, and B/NA have never been easier!
Reduce stress and delays in the seasonal strain update process with confidence that the VaxArray Influenza Seasonal NA Assay is confirmed reactivity for all WHO-recommended strains. The VaxArray Influenza Seasonal NA Multivalent Assay Kit is a powerful tool for rapid, multivalent detection of NA vaccine components with high specificity.
VaxArray Influenza Pandemic Hemagglutinin Multivalent Assay
Streamline the development of pandemic influenza vaccines with the VaxArray Influenza Pandemic HA Assay Kit that targets H5, H7, and H9 subtypes. Each test contains 5 capture antibodies that target the most prominent and potentially pandemic A/H5 viruses, 2 capture antibodies for broad reactivity with H7, and 2 antibodies recognizing H9. This assay has been verified reactive with an array of strains and production methods from the following subtypes:
- H5N1, H5N8, H5N6, and H5N2
- H7N2, H7N3, H7N7, H7N9
- H9N2
Publications
Byrne-Nash, R.T., et al. VaxArray potency assay for rapid assessment of “pandemic” influenza vaccines. 2018. NPJ Vaccines. 3:43; doi:10.1038/s41541-018-0080-6
Kuck L.R., et al. VaxArray assessment of influenza split vaccine potency and stability. 2017. Vaccine. 15(35) 1918-1925. doi:10.1016/j.vaccine.2017.02.028
Fox, A., et al. Opposing effects of prior infection versus prior vaccination on vaccine immunogenicity against influenza A(H3N2) viruses. 2022. Viruses. 14(3):470. doi:10.3390/v14030470
Byrne-Nash, R.T., et al. A neuraminidase potency assay for quantitative assessment of neuraminidase in influenza vaccines. 2019. NPJ Vaccines. 4:3; doi:10.1038/s41541-019-0099-3
Centi, C., et al. VaxArray seasonal influenza assessment of adjuvant-containing vaccine formulation. Poster presented at World Vaccines Conference 2017.
Byrne-Nash, R.T., et al. VaxArray assessment of influenza vaccine potency and stability. Poster presented 2016.