Introducing 5’CapQ
Accelerate Development of mRNA Vaccines
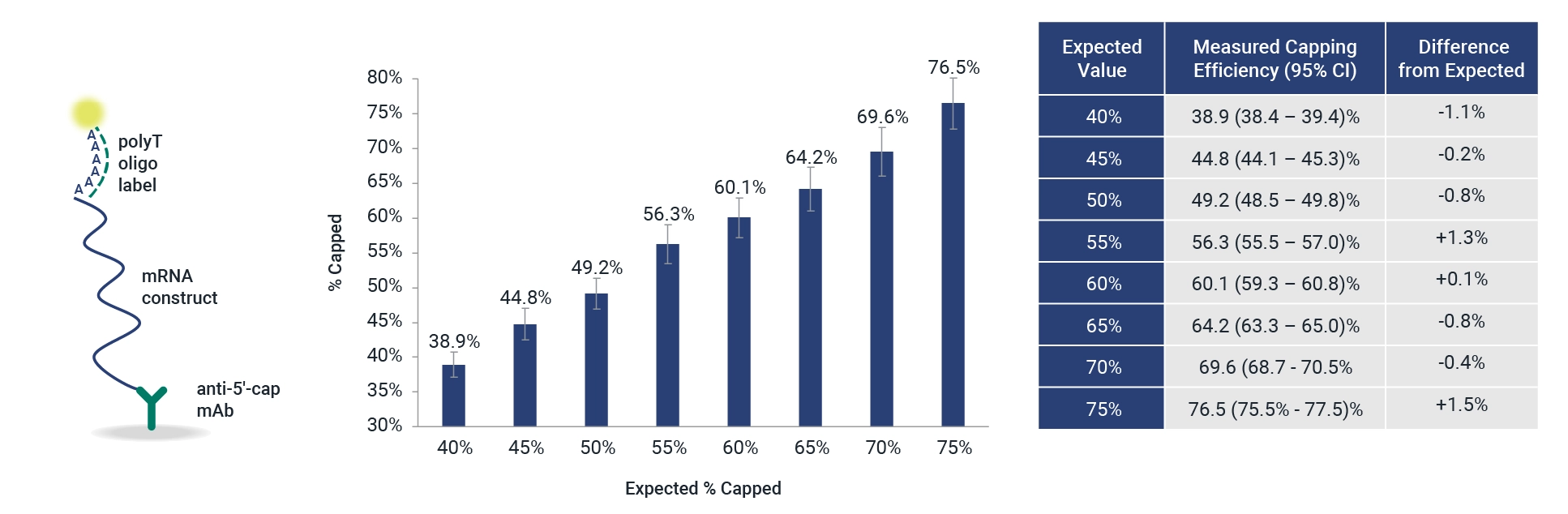
InDevR’s latest mRNA product—the 5’CapQ Assay—is a novel, leading technology that enables measurement of % capped and intact mRNA.
Vaccine manufacturers measure 5′ capping efficiency using complex and indirect chromatographic methods that can take 1-2 days with additional queue and analysis time. Paired with the easy-to-use VaxArray Platform, the 5’CapQ Assay eliminates the need for highly trained experts and reduces these analytical turnaround times during mRNA vaccine development. With this assay:
- Develop vaccines faster – Consolidate your mRNA capping and intactness testing into 1 assay that can be completed in less than two hours.
- Bring mRNA analytics directly to your facility – The VaxArray Platform reduces testing bottlenecks by enabling analysis right at your benchtop.
- Standardize your mRNA vaccine characterization – Easy-to-use microarray-based platform that requires no unique expertise to operate.
| Characteristic | Technique |
|---|---|
| RNA concentration | RT-PCR |
| Ultraviolet Spectroscopy (UV) | |
| mRNA intactness (size & length) |
Capillary electrophoresis (CE) / Capillary gel electrophoresis (CGE) |
| Agarose gel electrophoresis | |
| mRNA intactness (cap & tail) |
InDevR’s VaxArray 5’CapQ |
| 5’ capping efficiency | InDevR’s VaxArray 5’CapQ |
| High-performance liquid chromatography (HPLC) | |
| Liquid chromatography mass spectroscopy (LC-MS/MS) | |
| 3’ poly(A) tail length | High-performance liquid chromatography (HPLC) |
| Liquid chromatography mass spectroscopy (LC-MS/MS) |