Introducing OmniFlu HA/NA 96
Speed Development of Seasonal Influenza Vaccines
HA and NA Quantification in One Assay
In addition to hemagglutinin (HA), neuraminidase (NA) is increasingly being considered for inclusion in influenza mRNA vaccines to increase immunogenicity, and may be present in both egg-based and cell-based vaccines. Regulatory agencies, at minimum, require NA identity testing for NA-containing vaccines and discussions are ongoing regarding future regulation of NA content, with many manufacturers preferring additional characterization of NA in their vaccine products.
OmniFlu HA/NA is a ready-to-go, pre-validated, automation-friendly solution for seasonal influenza vaccine manufacturers. It consolidates hemagglutinin and neuraminidase detection and quantification into one assay. It drastically reduces testing time and eliminates in-house development, optimization, and validation work upfront.
- More answers per test — Quantify 7 seasonal HA and NA influenza targets in a single assay.
- Pre-validated each season — Validated for use with every World Health Organization-recommended seasonal influenza strain.
- Broad reactivity — Reactive with egg- and cell-based vaccines, recombinant antigens, and expressed HA/NA in cell-based assays for mRNA vaccines.

OmniFlu HA/NA Microarray Layout
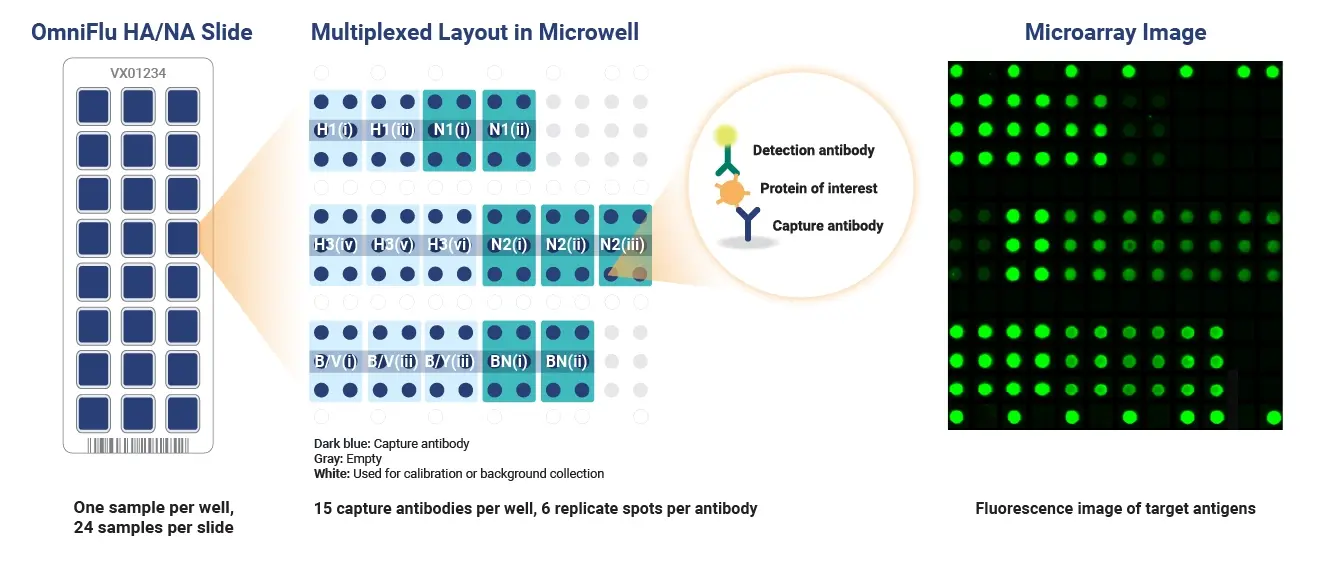
The OmniFlu HA/NA 96 targets 7 unique proteins (influenza HA from H1, H3, B/Victoria, and B/Yamagata, and influenza neuraminidase subtypes N1, N2, and B/NA) but contains multiple antibodies for each seasonal influenza subtype and lineage (except for B/Yamagata). This better ensures reactivity of at least one capture antibody per subtype or lineage depending on production method and strain.
Streamline Your Production
OmniFlu HA/NA 96 is only one of the innovative kits designed for use on the VaxArray® platform. This easy-to-use benchtop device enables vaccine developers and manufacturers to simplify and standardize their workflows. Explore additional kits and learn how the VaxArray platform can help you optimize your production line by visiting indevr.com/vaxarray
Product Details
| Description | Specification |
|---|---|
| Format | 24 Chamber Microarray Slide |
| Kit Size | 4 Slides, 96 Samples and/or Standards |
| Compatible Production Platform | Egg-based Cell-based (MDCK) Recombinant (Sf9) mRNA-expressed (HEK-293, Hep3b) |
| Target HAs | H1, H3, B/Victoria, B/Yamagata |
| Target NAs | N1, N2, and B/NA |
| Seasonal Reactivity Assessment | Reactive with most recent WHO strain recommendations (egg and cell) for Northern and Southern Hemispheres |
| Time to Result | < 2 hours |
| Instrument | VaxArray |
| Software | Version 3.X |







