nuIQ Assays
Simplify analysis of multivalent mRNA identity and quantity with 1 assay
Accelerate mRNA Vaccine and Biomarker Development with Multiplexed Custom Assays
mRNA vaccine and therapeutic development have relied on traditional CQA methods, with multiple techniques to properly analyze the identity and quantity of mRNA constructs. These techniques, including RT-PCR, sequencing (either NGS or Sanger), and UV spectroscopy, can be inefficient and cumbersome.
InDevR’s latest VaxArray Assay offering—nuIQ Assays—enables multiplexed analysis of construct-specific mRNA identity and quantity from multivalent mRNA vaccines in a single assay. With nuIQ, you can:
- Speed up vaccine or biomarker development – Consolidate your mRNA identity and quantity analysis to 1 assay and achieve results in less than two hours.
- Eliminate steps – No mRNA extraction, enzymatic digestion, primers, or amplification required.
- Simultaneously assess identity and quantity – Quantify multiple constructs simultaneously, and pair with unique custom detection labels for even higher information content.
- Standardize your mRNA vaccine characterization – Validated and standardized kits enable QC departments to easily achieve global quality initiatives.
Flexible Custom Assay Development
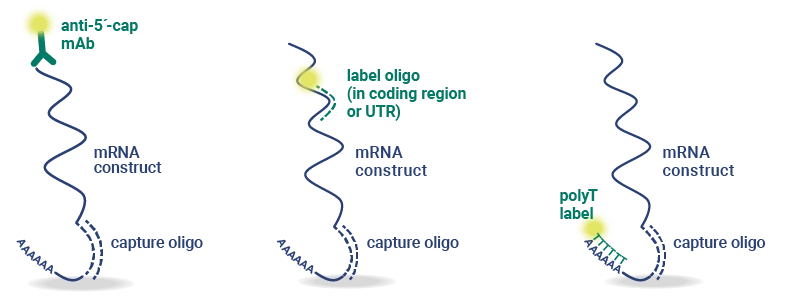
Work with InDevR’s Expert Services team to custom-design your nuIQ assay to analyze constructs for novel mRNA vaccines and therapeutic development. The oligo captures and labels are designed for target specificity to assess identity and quantity even in multivalent mixtures.
Multivalent mRNA Quantification
- Multiplexable microarrays deliver more results per assay
- Construct-specific labels and multiple captures per construct provide rich information content
nuIQ Assay Development Process


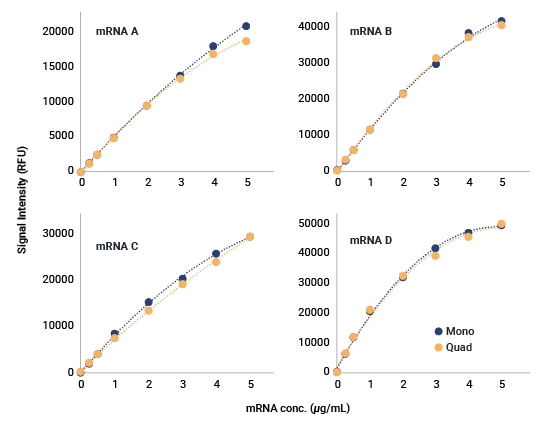
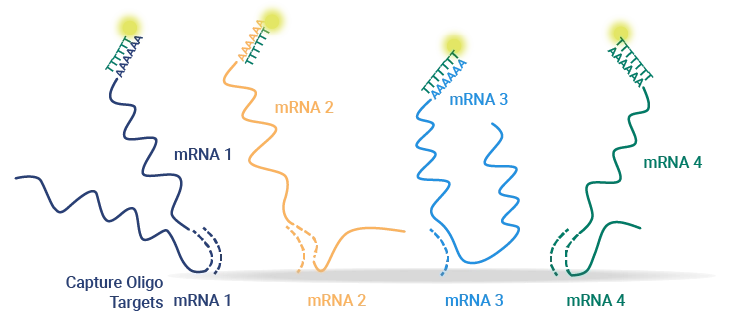 Capture oligos were designed to simultaneously test a mixture of four Pfizer-designed mRNA constructs in a quadrivalent vaccine under development.
Capture oligos were designed to simultaneously test a mixture of four Pfizer-designed mRNA constructs in a quadrivalent vaccine under development.