Bring Vaccines to Market Faster
Trusted Vaccine Industry Partner for Over 20 Years
World Vaccine Congress | Europe
October 28-31, 2024
Barcelona, Spain
Accelerate and simplify mRNA CQA testing: a novel strategy for rapid measurement of capped mRNA integrity and 5’ capping efficiency

Rosaria Esposito, PhD
Global Field Scientist VaxArray
bioMérieux
rosaria.esposito@biomerieux.com
InDevR presents a Technology Talk
in partnership with bioMérieux
October 29, 13:55 CEST
The 5′ cap and intact poly(A) tail structures of mRNA are important for protein expression and mRNA stability. The indirect chromatographic methods often used to measure 5’capping and polyA tailing efficiency can take 1-2 days, with additional queue and analysis time to measure mRNA concentration, intactness, and purity. In this talk you’ll learn about the 5’CapQ Assay, a novel strategy for the rapid measurement of intact, capped, and tailed mRNA in a single assay, with the VaxArray® system, an easy-to-use microarray-based platform.
High-Throughput, Multiplexed Analysis of HA and NA Content Across All Influenza Vaccine Production Platforms

Randy Lacey, PhD
Field Application Scientist
InDevR
Manufacturing Technologies
October 31, 9:45 CEST
Influenza vaccine production is a complex and challenging process that requires effective analytical tools for identifying and quantifying target antigens throughout drug development and production. Traditional analytical tools such as SRID and ELISA can be effective but have limitations in both throughput and capacity for multiplexed analysis. In this talk, you’ll learn about how InDevR’s VaxArray platform and the recently released OmniFlu HA/NA 96 Assay provide solutions to these analytical hurdles allowing for high throughput, multiplexed analysis of seasonal HA and NA proteins from all influenza vaccine production platforms
OmniFlu HA/NA 96 Assay for Seasonal Influenza Vaccines
InDevR’s VaxArray OmniFlu HA/NA 96 assay consolidates HA and NA detection and quantification into one simple assay. OmniFlu HA/NA is a ready-to-go, pre-validated, automation-friendly solution for seasonal influenza vaccine manufacturers. OmniFlu HA/NA is a ready-to-go, pre-validated, automation-friendly solution for seasonal influenza vaccine manufacturers.
- Achieve More Results Per Test – Quantify 7 seasonal HA and NA influenza targets (including influenza HA from H1, H3, B/Victoria and B/Yamagata, and influenza neuraminidase subtypes N1, N2, and B/NA) in a single assay.
- Validated Each Season – Validated for use with every World Health Organization-recommended seasonal influenza strain.
- Broad Reactivity – Reactive with egg- and cell-based vaccines, recombinant antigens, and expressed HA/NA in cell-based assays for mRNA vaccines.
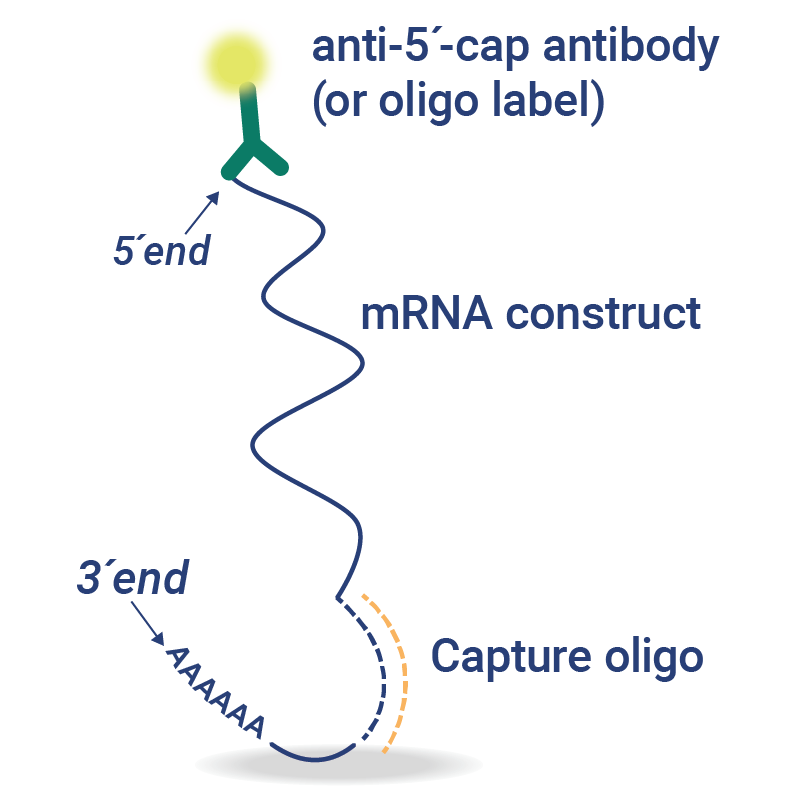
5’CapQ Assay: Smplify Measurement of mRNA Attributes
The 5′ cap and poly(A) tail elements of mRNA are essential for protein expression and mRNA stability. Waiting for the mRNA analysis of concentration, intactness, and purity from a central laboratory or outsourced partner slows down mRNA vaccine development.
In just 90 minutes, the 5’CapQ Assay is a simple, direct measurement of capped and tailed mRNA. This assay works by capturing the mRNA molecules via the 5’ cap and labeling the molecules on the poly(A) tail, enabling quantification of only the complete intact mRNA molecules. RNA molecules that lack a 5’ cap or poly(A) tail are not measured in the 5’CapQ Assay, leading to a single test for quantifying your mRNA.
- Develop Vaccines Faster – Consolidate your mRNA capping and intactness testing into 1 assay that can be completed in less than two hours.
- Analyze at Your Benchtop – Reduce testing bottlenecks by bringing mRNA analytics to your facility.
- Simplify Workflow Process – compatible with LNP formulations eliminating the need for laborious extraction procedures.