With the recent multistate outbreak of the highly pathogenic avian influenza A(H5N1) virus in dairy cows and other animals, the CDC is monitoring the spread of the virus between animals and people. In two initial human cases of H5 avian flu reported from the recent dairy cow outbreak, individuals were exposed at independent dairy farms with ongoing outbreaks. Symptoms were limited to eye infections. “Conjunctivitis (eye infection) has been associated with previous human infections with avian influenza A viruses and is part of the current CDC case definition for A(H5N1) surveillance.”1,2
The USDA, HHS, and CDC have identified $194 million in combined funds to continue work on testing, prevention, and treatment for H5N1. The CDC is taking several steps, including providing funding for ongoing testing and surveillance, supporting the ability to surge testing efforts, and manufacturing diagnostic kits for virologic surveillance. In addition, $8 million has been set aside to evaluate the effectiveness of current Candidate Vaccine Viruses (CVVs) and develop new ones if needed.3
With this potential pre-pandemic threat, it’s important to stay ahead and quickly develop novel vaccines to prevent another global pandemic. To this effort, Curevac recently announced a collaboration with GSK to begin Phase 1 clinical trials of an investigational influenza A (H5N1) vaccine candidate.4
InDevR’s Expertise Accelerates Your Vaccine Development Time to Market
With over two decades of experience in custom vaccine assay development, and a suite of sophisticated assay tools and curated reagents, InDevR has efficient solutions to expedite your vaccine development process.
InDevR’s VaxArray® Platform is an automated, microarray-based multiplex immunoassay solution for vaccine testing that consolidates multiple assays into a single test. It’s more sensitive and specific than traditional methods like SRID, and significantly reduces time and resources needed, enabling faster development, optimization, and production scale-up during critical times.
Streamline Development of Pandemic Influenza HA Vaccines
VaxArray Influenza Pandemic HA Assay Kit is confirmed reactive with the circulating H5 strain (A/dairy_cattle/Texas/2024) and pre-validated with the most prominent and potentially pandemic A/H5 viruses, reducing your analytical development burden and accelerating your path to market. The following subtypes were used to validate this HA assay: H5N1, H5N8, H5N6, H5N2, H7N2, H7N3, H7N7, H7N9, and H9N2.
Byrne-Nash, R.T., et al. VaxArray potency assay for rapid assessment of “pandemic” influenza vaccines. 2018. NPJ Vaccines. 3:43; doi:10.1038/s41541-018-0080-6
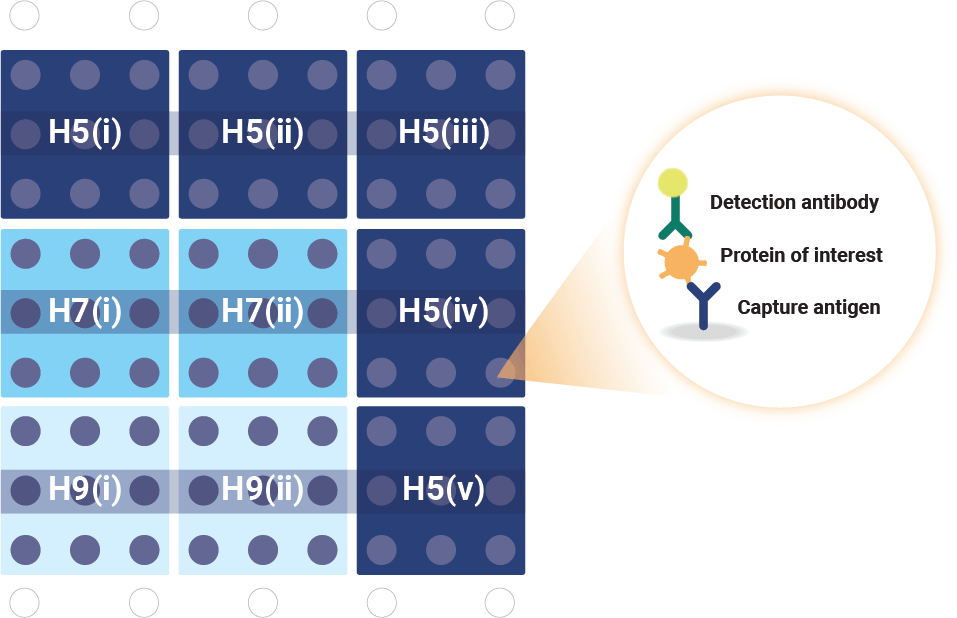
VaxArray Influenza Pandemic HA Multivalent Assay. The microarray contains nine replicate spots of each monoclonal antibody.
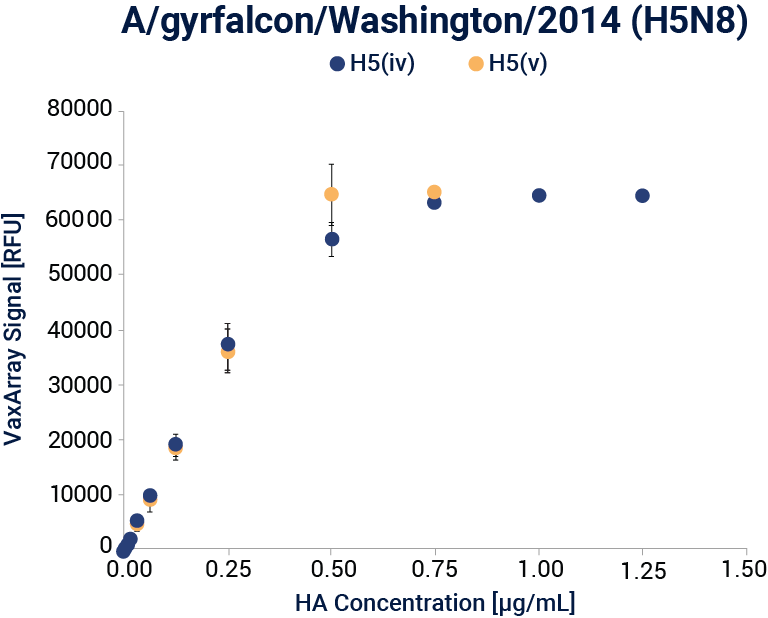
Linear dynamic range response curves for A/H5N8 A/gyrfalcon/Washington/41088-6/2014 (IRR, FR-1418) antigens for each corresponding capture antibody are shown. Error bars represent the standard deviation of the 9 antibody spots for the corresponding capture antibody for each array.
NA Identity and Quantity in One Simple Test
Neuraminidase (NA) identity testing is required for any vaccines expected to contain NA, and NA quantification is an area of active interest for future regulation. The VaxArray Influenza Seasonal NA Multivalent Assay Kit is available immediately for identity and quantity of N1 from the circulating H5N1 strain, speeding up your vaccine development timeline.
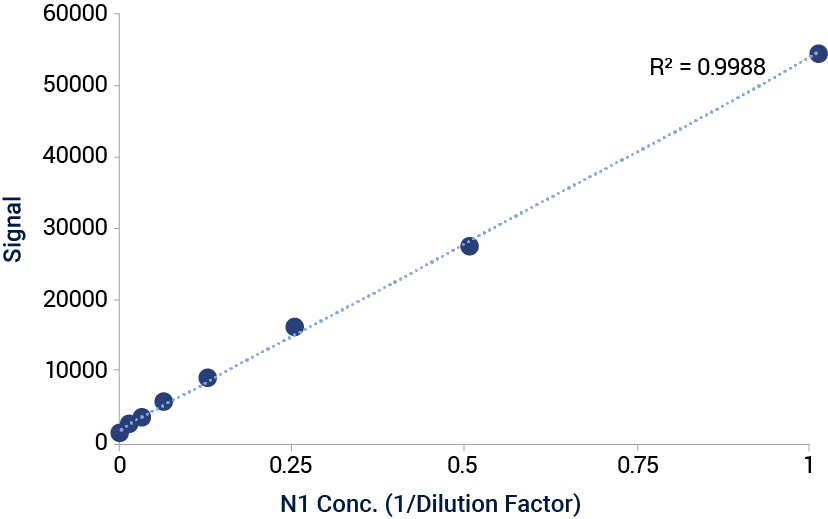
Signal VaxArray Influenza Seasonal NA Multivalent Assay. Serial dilution of cell lysates containing HEK-expressed N1 antigen from A/dairy_cow/Texas/24-008749-001-original/2024 on the VaxArray N1(i) capture antibody.
Custom Serological Assays for Clinical Trials or Pre-Existing Immunity
Conventional serological characterization assays often pose challenges in terms of complexity, time consumption, and resource requirements, particularly when assessing antibody titers against multiple targets. InDevR’s Expert Services Team has vast experience in influenza serological testing and will simplify and streamline your analytical development, enabling the generation of multiple results from a single test.
Contact Us
References
- National Center for Immunization and Respiratory Diseases (NCIRD) (2024, May 10), CDC A(H5N1) Bird Flu Response Update, U.S. Department of Health and Services. Accessed 25 May 2024, https://www.cdc.gov/flu/avianflu/spotlights/2023-2024/bird-flu-response-update.html#:~:text=The%20second%20human%20case%20of,a%20Person%20in%20the%20U.S.
- National Center for Immunization and Respiratory Diseases (NCIRD) (2024, May 22), CDC Reports Second Human Case of H5 Bird Flu Tied to Dairy Cow Outbreak, U.S. Department of Health and Services. Accessed 25 May 2024, https://www.cdc.gov/media/releases/2024/s0522-human-case-h5.html
- National Center for Immunization and Respiratory Diseases (NCIRD) 2024, May 10), Fact Sheet: USDA, HHS Announce Actions to Reduce Impact and Spread of H5N1, U.S. Department of Health and Services. Accessed 25 May 2024, https://www.hhs.gov/about/news/2024/05/10/fact-sheet-usda-hhs-announce-new-actions-reduce-impact-spread-h5n1.html
- CureVac (2024, April 24), CureVac Announces Start of Combined Phase 1/2 Study in Avian Influenza (H5N1); Development in Collaboration with GSK. Accessed 25 May 2024, https://www.curevac.com/en/curevac-announces-start-of-combined-phase-1-2-study-in-avian-influenza-h5n1-development-in-collaboration-with-gsk/
- Byrne-Nash, et al. VaxArray potency assay for rapid assessment of “pandemic” influenza vaccines. npj Vaccines (2018) 3:43; doi:10.1038/s41541-018-0080-6 https://www.nature.com/articles/s41541-018-0080-6