Bring Vaccines to Market Faster
Validated and standardized kits enable QC departments to quickly achieve global quality initiative
World Vaccine Congress
April 22-24, 2025
Washington, D.C.
Visit Us at Booth #471
Flu Wars: The Assay Strikes Back

Brian K Nunnally, Ph.D.
Vice President and Enterprise Head of Quality Control for CSL
InDevR presents a Technology Talk
in partnership with CSL
April 21, 10:00 ET
In 1979, the World Health Organization recommended the use of a new assay for flu content known as SRID, a standard potency test that served the world for 45 years. While SRID was a useful tool, it required the creation of special reagents for each new sub-type of the virus strain, which was time-consuming and can delay vaccine availability. As the need for quicker and better assays grew, the search for new techniques to replace SRID began. The ideal SRID replacement should be a biologic assay capable of quantitating multiple sub-types, including alternate clades of the same sub-type, be stability indicating, immediately available, precise, accurate, simple to run, and cost-effective. Several potential replacements are being considered, including antibody-based assays, surface plasmon resonance-based assays, and bio-IDMS-based assays. Each technique has its own advantages and limitations, and the presentation will explore the viability of these methods in ensuring the safety and health of the world’s inhabitants.
Maximize Efficiency in Vaccine Development with VaxArray
Discover the VaxArray platform, your solution in multiplexed immunoassays designed to streamline vaccine development and analysis. This state-of-the-art system offers unparalleled precision and efficiency, empowering researchers to achieve faster, more reliable results in their efforts to advance public health.
- High-Efficiency Testing – improve at-line analysis with multiplexed immunoassays.
- Simplify Standardization – 21 CFR Part 11 compatible software and easy-to-use kits simplify standardization across workflows.
- Versatile Platform – analyze a wide range of vaccine components, from antibodies and antigens to nucleic acids.
OmniFlu HA/NA 96 Assay: Standardize HA/NA Testing in a Single Assay
InDevR’s VaxArray OmniFlu HA/NA 96 assay consolidates HA and NA detection and quantification into one simple assay. OmniFlu HA/NA is a ready-to-go, pre-validated, automation-friendly solution for seasonal influenza vaccine manufacturers. OmniFlu HA/NA is a ready-to-go, pre-validated, automation-friendly solution for seasonal influenza vaccine manufacturers.
- Achieve More Results Per Test – Quantify 7 seasonal HA and NA influenza targets (including influenza HA from H1, H3, B/Victoria and B/Yamagata, and influenza neuraminidase subtypes N1, N2, and B/NA) in a single assay.
- Validated Each Season – Validated for use with every World Health Organization-recommended seasonal influenza strain.
- Broad Reactivity – Reactive with egg- and cell-based vaccines, recombinant antigens, and expressed HA/NA in cell-based assays for mRNA vaccines.
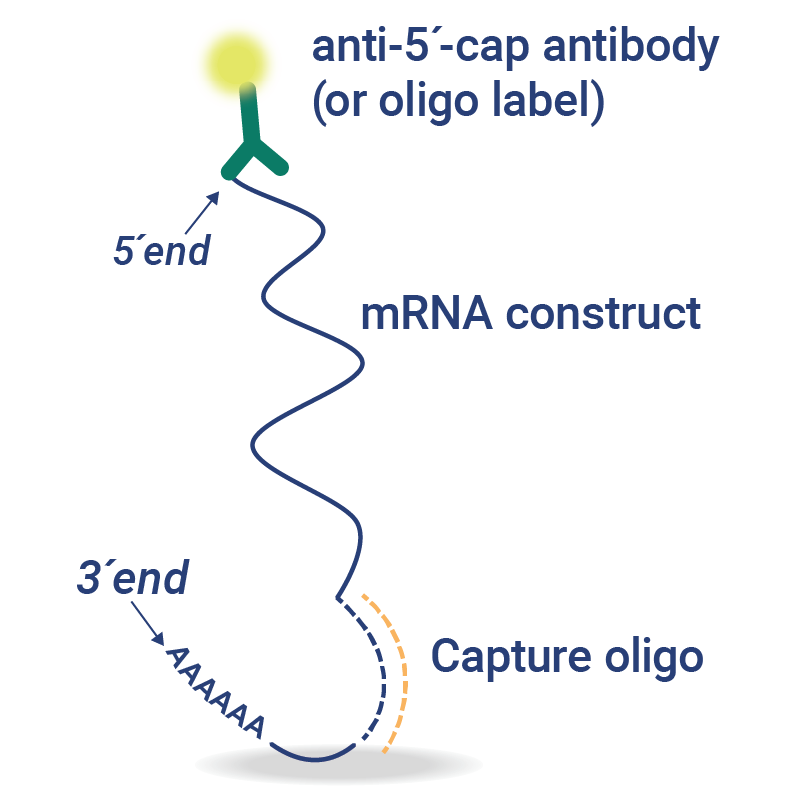
5’CapQ Assay: Simplify Measurement of mRNA Attributes
The 5′ cap and poly(A) tail elements of mRNA are essential for protein expression and mRNA stability. Waiting for the mRNA analysis of concentration, intactness, and purity from a central laboratory or outsourced partner slows down mRNA vaccine development.
In just 90 minutes, the 5’CapQ Assay is a simple, direct measurement of capped and tailed mRNA. This assay works by capturing the mRNA molecules via the 5’ cap and labeling the molecules on the poly(A) tail, enabling quantification of only the complete intact mRNA molecules. RNA molecules that lack a 5’ cap or poly(A) tail are not measured in the 5’CapQ Assay, leading to a single test for quantifying your mRNA.
- Develop Vaccines Faster – Consolidate your mRNA capping and intactness testing into 1 assay that can be completed in less than two hours.
- Analyze at Your Benchtop – Reduce testing bottlenecks by bringing mRNA analytics to your facility.
- Simplify Workflow Process – compatible with LNP formulations eliminating the need for laborious extraction procedures.