Bring Vaccines to Market Faster
Trusted Vaccine Industry Partner for Over 20 Years
World Vaccine Congress
April 1-4, 2024
Washington, D.C.
Visit Us at Booth #559

Dr. Izabela Ragan DVM, PhD
Director of Research and Development
Solaris Vaccines
Izabela.Ragan@solarisvax.com
The COVID-19 pandemic has underscored the importance of rapid vaccine development and manufacturing for emerging pathogens with significant global impacts. In addressing this challenge, we have utilized a novel photochemical method to produce whole-pathogen inactivated vaccine candidates, SolaVAX, for both COVID and influenza. The method combines UV light with riboflavin (Vitamin B2) to irreversibly damage nucleic acid while preserving key surface antigens. Our pre-clinical studies to date demonstrate improved preservation and antigenicity of viral proteins compared to traditional inactivation methods, provide significant neutralizing antibody response in animal models, as well as efficacy with the SolaVAX COVID vaccine to reduce viral replication and lung pathology in the hamster model. Integral to the development of SolaVAX has been the InDevR VaxArray® platform, a rapid, multiplexing technology that provides essential data on antigen integrity and quantification for the SolaVAX COVID and influenza vaccine development. The synergy between SolaVAX’s innovative whole-pathogen photochemical inactivation and VaxArray precise antigen quantification exemplifies a pioneering approach to vaccine development and manufacturing, offering a robust response to emerging infectious diseases.
Expert Services
InDevR’s Expert Services is a customized, efficient solution for vaccine manufacturers to help address and eliminate development bottlenecks and accelerate the analysis and validation.
- Expertly Customized Assays – save months developing, optimizing, and validating in-house assays.
- Multiplexed Assay Technology – enables quicker analysis over traditional single-plex methods like ELISA.
- Integrated Custom Solutions – simplify your development and manufacturing bioprocesses.
Maximize Efficiency in Vaccine Development with VaxArray
Discover the VaxArray platform, your solution in multiplexed immunoassays designed to streamline vaccine development and analysis. This state-of-the-art system offers unparalleled precision and efficiency, empowering researchers to achieve faster, more reliable results in their efforts to advance public health.
- High-Efficiency Testing – improve at-line analysis with multiplexed immunoassays.
- Simplify Standardization – 21 CFR Part 11 compatible software and easy-to-use kits simplify standardization across workflows.
- Versatile Platform – analyze a wide range of vaccine components, from antibodies and antigens to nucleic acids.
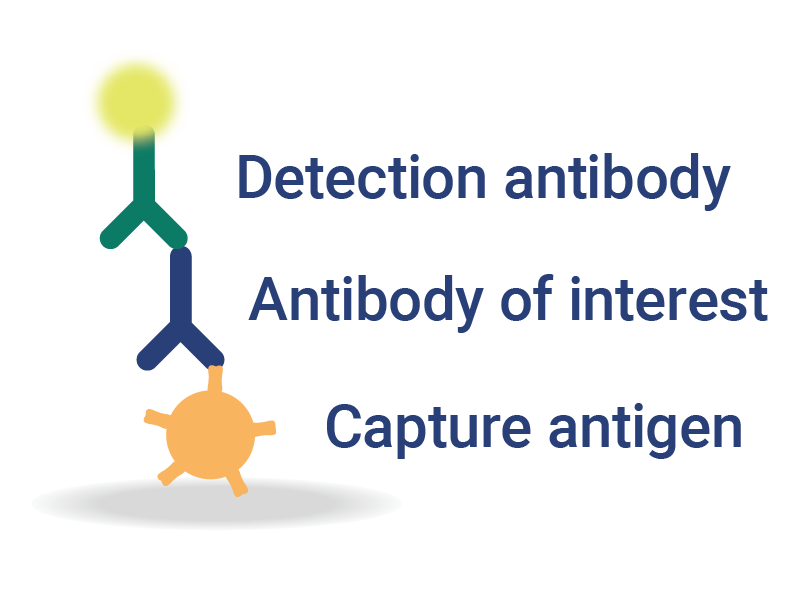
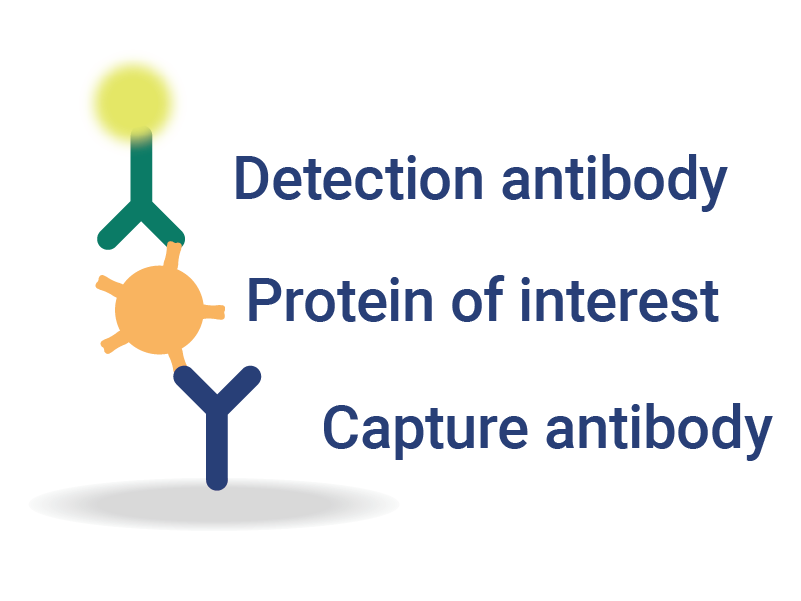
OmniFlu Multiplexed Assays for Subtype-Specific Influenza Analysis
InDevR’s VaxArray OmniFlu multiplex assay enables subtype-specific influenza vaccine quantification.
- Achieve Results Faster – fast and easy with <30 minutes of hands-on time and <1 hour to result.
- Highly Sensitive Assays – 100x more sensitive than SRID, with demonstrated equivalence for monovalent and multivalent vaccines.
- Expertly Designed Reagents – capture reagents are selected to be robust against antigenic drift.
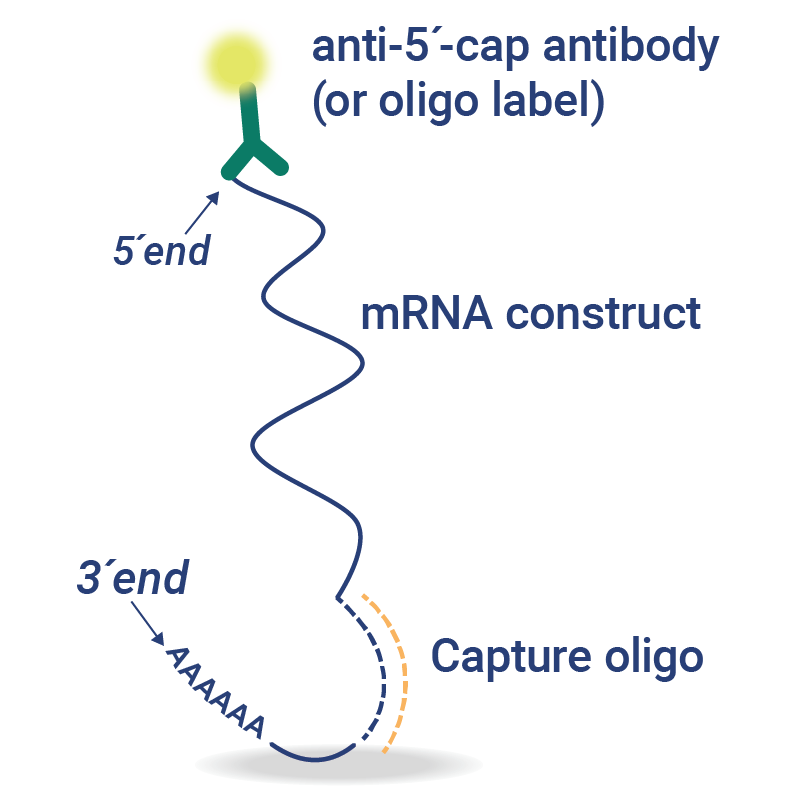
Custom VaxArray for mRNA
VaxArray assays can be customized to rapidly assess mRNA construct identity, quality, and cell-based expression from a single construct or multiplexed samples.
- Custom Multiplexed Assays – multiplexed assay simplifies the development of high-valency mRNA vaccines.
- Sequence-Specific Assays – sequence-specific capture oligos are designed for assessment of mRNA identity, quantity, and integrity.
- Simplify Workflow Process – compatible with LNP formulations eliminating the need for laborious extraction procedures.